29011000 SATURATED ACYCLIC HYDROCARBONS
A Saturated hydrocarbon is a hydrocarbon in which all the carbon-carbon bonds are single bonds. A hydrocarbon is an organic compound whose only constituents are carbon and hydrogen. As the name suggests, saturated hydrocarbons are hydrocarbons in which all the carbon atoms are bonded to four other atoms and are ‘saturated’, implying that no carbon-carbon multiple bonds exist in these organic compounds.
- Butane (C4H10)
- Octane (C8H18)
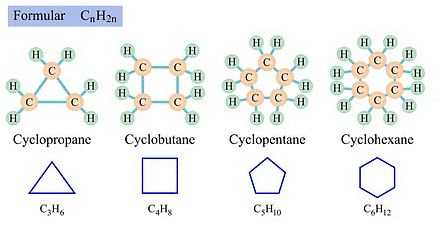
- Cyclohexane (C6H12)
- Cyclopropane (C3H6)
Cycloalkanes are alkanes that have a monocyclic ring structure. Since all the carbon-carbon bonds in cycloalkanes are single bonds, they are considered to be saturated hydrocarbons. Therefore, the general formula of a saturated hydrocarbon can be written as CnH(2n + 2 – 2r), where ‘r’ is the total number of rings in the molecule.
Types of Saturated Hydrocarbons
A saturated hydrocarbon can have a linear, branched, or ring-shaped structure and can, therefore, be classified into one of the following types:
- Alkanes
- Cycloalkanes
It is important to note that even polycyclic alkanes (alkanes with several rings in their structures) are also categorized as cycloalkanes and are, therefore, a type of saturated hydrocarbons.
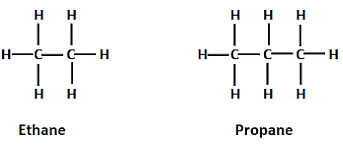
Alkanes
Cycloalkanes
APPLICATION
Alkanes are widely used as fuels, heating oils, and solvents. A few other uses of saturated hydrocarbons are listed below.
- Methane is used as a fuel in several automobiles, water heaters, and ovens. In its refined form, liquid methane can also serve as rocket fuel.
- Several cryogenic refrigeration systems use ethane as a refrigerant. It is also used in the production of ethylene.
- The propellant used in several aerosol sprays is a saturated hydrocarbon known as propane. This compound is also used as a fuel for hot air balloons.
- Octane is a very important component of gasoline since it helps prevent engine damage.
- Cycloalkanes also find use in motor fuels, diesel, petroleum gas, and other heavy oils.
- The manufacture of rubber and nylon also involves the use of cycloalkanes.

