Dichloroanilines are chemical compounds which consist of an aniline ring substituted with two chlorine atoms and have the molecular formula C6H5Cl2N. There are six isomers, varying in the positions of the chlorine atoms around the ring relative to the amino group. As aniline derivatives, they are named with the amino group in position 1. They are all colorless, although commercial samples can appear colored due to the presence of impurities. Several derivatives are used in the production of dyes and herbicides.
Dichloroaniline, which is an organic compound with the chemical formula C6H5Cl2N. It is a type of aniline in which two of the hydrogen atoms on the benzene ring are replaced by chlorine atoms. Dichloroaniline is used in the production of dyes, pigments, and other organic compounds.
In terms of physical properties, dichloroaniline is a crystalline solid with a melting point of around 47-48°C. It is soluble in organic solvents such as ethanol, acetone, and benzene, but insoluble in water.
Dichloroaniline is also known to be toxic and can have harmful effects on human health if ingested or inhaled. It is classified as a hazardous substance and should be handled with care.
In summary, Dichloroaniline is an important organic compound used in the production of various industrial products. Its physical properties make it a useful chemical, but its toxicity means that it needs to be handled with care.
Top of Form
Dichloroaniline is an organic compound with the molecular formula C6H5Cl2N. It is a colorless to yellowish crystalline solid that is sparingly soluble in water but soluble in organic solvents. Dichloroaniline belongs to the class of substituted anilines, which are organic compounds consisting of an aniline core (C6H5NH2) substituted with one or more chlorine atoms.
Dichloroaniline is used as an intermediate in the synthesis of various organic compounds, such as dyes, pigments, and pharmaceuticals. It is also used as an herbicide and pesticide.
In terms of safety, dichloroaniline is considered to be a hazardous substance, and exposure to it should be minimized. It can cause irritation to the skin, eyes, and respiratory tract, and prolonged exposure can lead to more serious health effects.
In conclusion, dichloroaniline is an important organic compound with various applications in industry and agriculture. However, precautions should be taken when handling and using it due to its potential hazards.
Compound Name
CAS
Chemical Structure
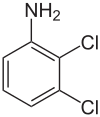
2,3-Dichloroaniline
608-27-5
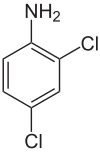
2,4-Dichloroaniline
554-00-7
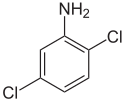
2,5-Dichloroaniline
95-82-9
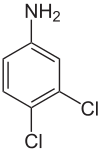
2,6-Dichloroaniline
608-31-1
3,5-Dichloroaniline
95-76-1
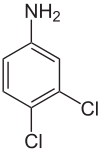
5,6-Dichloroaniline
626-43-7


