Cas no:
106-99-0
Einecs no: 203-450-8
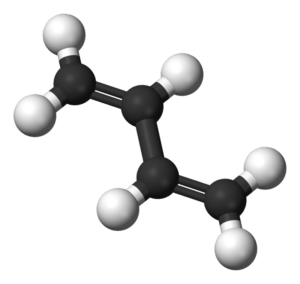
Formula: C4H6
Molar mass: 54.0916 g/mol
Boiling point: -4.4 °C
Density: 615 kg/m³
Buta-1, 3-diene is a hydrocarbon with a repeating butadiene unit. Butadiene is also made by the polymerization of buta-1, 3-diene, but with a catalyst. Butadiene is one of the most common synthetic rubbers, and is used in tires. Furthermore, butadiene is used in the production of synthetic rubber, butadiene acrylic acid copolymer, butadiene rubber, butadiene styrene rubber, and butadiene styrene butadiene rubber.
Although butadiene breaks down quickly in the atmosphere, it is nevertheless found in ambient air in urban and suburban areas as a consequence of its constant emission from motor vehicles.
Product Description
1,3-Butadiene is the organic compound with the formula (CH =CH It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two vinyl groups. It is the simplest conjugated diene.
Uses
Most butadiene is used to make synthetic rubbers for the manufacture of tyres, grommets and elastic bands.
The conversion of butadiene to synthetic rubbers is called polymerization, a process by which small molecules (monomers) are linked to make large ones (polymers). The mere polymerization of butadiene gives polybutadiene, which is a very soft, almost liquid material. The polymerization of butadiene and other monomers gives copolymers, which are more valued. The polymerization of butadiene and styrene and/or acrylonitrile, such as acrylonitrile butadiene styrene (ABS), nitrile-butadiene (NBR), and styrene-butadiene (SBR). These copolymers are tough and/or elastic depending on the ratio of the monomers used in their preparation. SBR is the material most commonly used for the production of automobile tyres. Precursors to still other synthetic rubbers are prepared from butadiene. One is chloroprene
Smaller amounts of butadiene are used to make adiponitrile, a precursor to some nylons. The conversion of butadiene to adiponitrile entails the addition of hydrogen cyanide to each of the double bonds in butadiene. The process is called hydrocyanation.
Butadiene is used to make the solvent sulfolane.
Butadiene is also useful in the synthesis of cycloalkanes and cycloalkenes, as it reacts with double and triple carbon-carbon bonds through Diels-Alder reactions. The most widely used such reactions involve reactions of butadiene with one or two other molecules of butadiene, i.e., dimerization and trimerization respectively. Via dimerization butadiene is converted to 4-vinylcyclohexene and cyclooctadiene. In fact, vinylcyclohexene is a common impurity that accumulates when butadiene is stored. Via trimerization, butadiene is converted to cyclododecatriene. Some of these processes employ nickel- or titanium-containing catalysts

